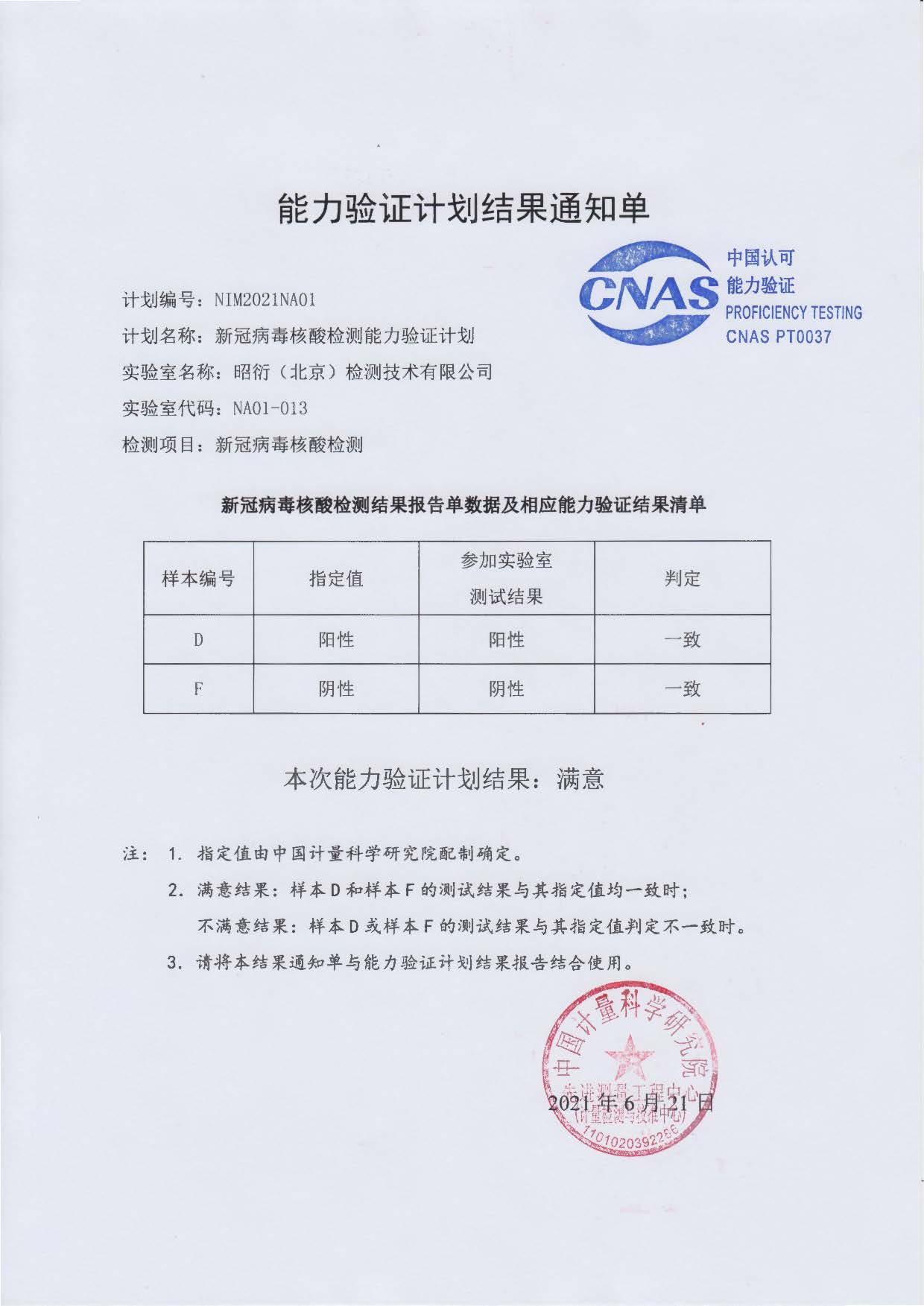
JOINN Medical Testing Successfully Passed the Verification of Novel Coronavirus Nucleic Acid Testing Ability
2021-06-23
Recently, JOINN Medical Testing Technology Co., Ltd., has successfully passed the verification of Novel Coronavirus nucleic acid testing ability. The results of the program are accredited by the China National Accreditation Service for Conformity Assessment (CNAS) and implemented by the National Institute of Metrology, China.
JOINN Medical Testing has built a competitive bioanalysis platform for cell and gene therapy products. The bioanalysis methods for qPCR and flow cytometry has been established and verified, and could meet the requirements of the latest white paper and literature [1-6], and are in line with world-class laboratories.
JOINN has undertook more than 45% safety evaluations of gene and cell therapy products in China, including the distribution, homing and abscission of gene therapy products (AAV, ADV, siRNA, oncolytic viruses such as Sendai virus, VSV, HSV, poxvirus, etc.). Tissue distribution and cell subtype changes of cell therapy products (CAR-T, TCR-T, CTL, stem cells, etc.).
