Service Capability
JOINN has over 25 years of experience in drug safety evaluation, and has rich experience. By adopting international (FDA, ICH) technical standards, personalized trial design, and standardized quality management (GLP), JOINN can provide evaluation reports that meet regulatory (GLP) requirements to NMPA of China, FDA of the United States, and other countries.
About joinn
One-stop Solutions for Your Drug Innovation
From Early-stage R&D to Cllinical Trials
From Early-stage R&D to Cllinical Trials
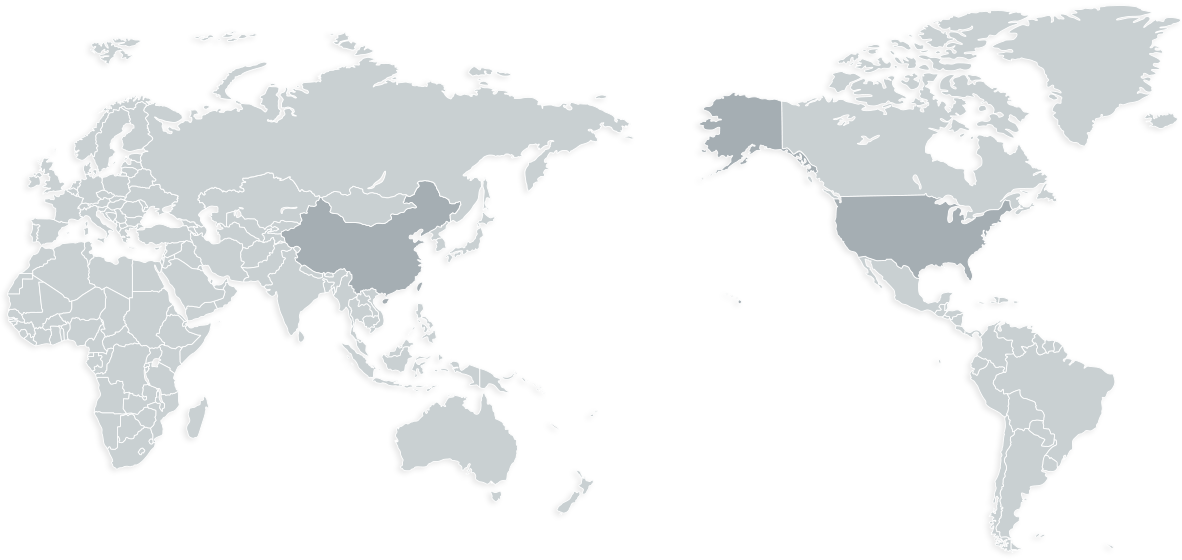
Beijing
JOINN Laboratories (China) Co., Ltd.
JOINN Medsafe Co., Ltd.
JOINN Clinical (Beijing) Co., Ltd.
JOINN Biologics (Beijing) Co., Ltd.
JOINN Medical Testing Laboratories (Beijing) Co., Ltd.
JOINN Medsafe Co., Ltd.
JOINN Clinical (Beijing) Co., Ltd.
JOINN Biologics (Beijing) Co., Ltd.
JOINN Medical Testing Laboratories (Beijing) Co., Ltd.
Suzhou
JOINN Laboratories (Suzhou) Inc.
JOINN Clinical (Suzhou) Co., Ltd.
JOINN Qichen Biotechnology (Suzhou) Co., Ltd.
Californi
JOINN Laboratories (CA) Co., Ltd.
Massachusetts
JOINN Biomere
Guangzhou
JOINN Laboratories (Guangzhou) Inc.
Wuzhou
JOINN Laboratories (Wuzhou), Co., Ltd
JOINN Biotechnology (Wuzhou) Co., Ltd.
Chongqing
JOINN Laboratories (Chongqing) Inc.